What Upstaza’s Likely Approval in Europe Means to Our Community

Chimes and notifications began to appear on my phone on the morning of May 20. Messages and questions in my inboxes soon followed.
Everyone was excited that the previous day, the Committee for Medicinal Products for Human Use (CHMP), an arm of the European Medicines Agency (EMA), had recommended approval of the gene therapy Upstaza (eladocagene exuparvovec) to treat aromatic l-amino acid decarboxylase (AADC) deficiency in the European Union.
As AADC News‘ Marta Figueiredo reported, the European Commission usually accepts the CHMP’s recommendations, meaning that Upstaza likely will become the first treatment for AADC deficiency. It is expected to be approved by the commission in about two months.
As the news broke, the question on everyone’s mind was, “What does this mean for us?”
Since AADC deficiency was first identified in 1990 — more than 30 years ago — the situation finally seems to be improving, and momentum is gaining.
As a parent advocate for the AADC deficiency community with a daughter who has benefited from Upstaza — formerly known as PTC-AADC — as part of a clinical trial, we were honored to share our story with the EMA and play a small part in helping to make this treatment become available.
The CHMP’s decision entails several positives. There are, however, some things to keep in mind. First, the CHMP provided a positive opinion of the treatment, but potential approval would happen for patients in Europe under exceptional circumstances, a type of authorization “granted to medicines where the applicant is unable to provide comprehensive data on the efficacy and safety under normal conditions of use, because the condition to be treated is rare or because collection of full information is not possible or is unethical,” according to an explanation by the EMA.
I was a little concerned when I read this detail. However, the EMA states that it is common for rare conditions that cannot undergo the standard rigorous testing and compliance protocols due to the low incidence rate.
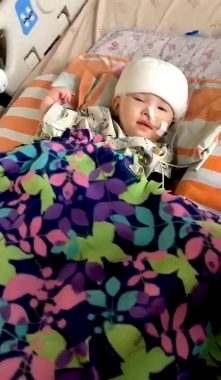
Our daughter, Rylae-Ann, undergoes gene therapy during a clinical trial for eladocagene exuparvovec. (Photo by Richard E. Poulin III)
Could global acceptance follow?
The hope is that the CHMP’s favorable opinion could lead to similar positive news in the U.K. and the U.S., and then other countries as well. This is crucial, as we know that global cases of AADC deficiency exist. And as I pointed out in a previous column, while only about 135 cases have been described globally since 1990, that number likely is much higher, perhaps as high as 1,800, according to one expert.
Innovation and competition
In my opinion, this development could also open the door for more competition and innovation in the area of treatments. Upstaza’s developer, PTC Therapeutics, has collected and shared data on the potential life-changing effects of gene therapy, including at the Virtual ISPOR Europe 2020 Conference. Additionally, everyone in our community saw the fantastic benefits of gene therapy in social media posts by families who were fortunate enough to join the clinical trials.
I wouldn’t be surprised if other pharmaceutical companies and medical device manufacturers took notice of this recent announcement. For example, innovation is already being studied in a small Phase 2 clinical trial testing the ability of ClearPoint’s Navigation System and SmartFlow Cannula to improve the delivery of the gene therapy.
In 2019, our daughter, Rylae-Ann, had gene therapy via the traditional stereotactic method. Now, in 2022, innovations are already in the pipeline, and more improvements likely could follow.
Greater awareness
I have never before received so many Google alerts for articles about AADC deficiency in such a short amount of time. It feels incredible that our small community now has a global spotlight on it. By maintaining this awareness, we can push for more research and additional treatments. Awareness can bring more funding to help families cover the cost of treatment and other expenses associated with receiving it, such as travel costs.
Hope for all rare diseases
AADC deficiency is not the only rare disease, of course. Last October, on AADC Deficiency Awareness Day, I explained how the National Organization for Rare Disorders lists more than 1,200 rare diseases. About 30 million people in the U.S. — about one out of 10 people — have a rare disease. Plus, an estimated 95% of rare diseases have no treatment available.
PTC Therapeutics’ success with gene therapy just might enable researchers to apply it to other rare diseases as well. Gene therapy already was a buzzword, and interest in it will continue to grow.
The fact that my friends in the AADC deficiency community and in other rare disease communities are on the verge of receiving treatment gives me so much pleasure. The future of medicine is here, and there is real hope for the families that follow.

Upstaza’s likely approval in Europe brings hope to the AADC deficiency community. (Photos by Richard E. Poulin III)
Note: AADC News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of AADC News or its parent company, BioNews, and are intended to spark discussion about issues pertaining to aromatic l-amino acid decarboxylase deficiency.







Comments