Kebilidi (eladocagene exuparvovec-tneq) for AADC deficiency
Last updated Nov. 19, 2024, by Marisa Wexler, MS

What is Kebilidi for AADC deficiency?
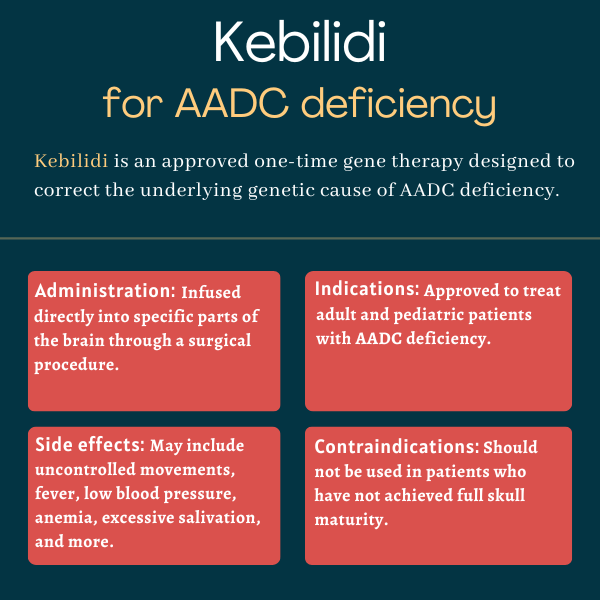
Kebilidi (eladocagene exuparvovec-tneq) is an approved one-time gene therapy designed to correct the underlying genetic cause of aromatic L-amino acid decarboxylase (AADC) deficiency.
Developed by PTC Therapeutics, which acquired the therapy in 2018 from Agilis Biotherapeutics, its initial developer, Kebilidi is administered directly into the brain via a minimally invasive neurosurgical procedure.
Kebilidi’s approval by the U.S. Food and Drug Administration (FDA) in 2024 made it the first approved disease-modifying therapy for AADC deficiency in the U.S. The therapy also is approved in the European Union and in the U.K., where it is sold under the name Upstaza.
Therapy snapshot
| Brand name: | Kebilidi |
| Chemical name: | Eladocagene exuparvovec-tneq |
| Usage: | Used to address the genetic defect that causes AADC deficiency |
| Administration: | Given directly into the brain via a surgical procedure |
How does Kebilidi work?
AADC deficiency is caused by mutations in the DDC gene, which provides instructions for making the AADC enzyme. This enzyme is needed to produce certain neurotransmitters, or signaling molecules, that nerve cells use to communicate with each other and the rest of the body. Two such neurotransmitters are serotonin and dopamine.
DDC mutations result in deficient levels of a working AADC enzyme, which means that the brain cannot make enough serotonin and dopamine, leading to hallmark neurological disease symptoms.
Kebilidi is a one-time gene therapy that uses a modified and harmless virus, called adeno-associated virus serotype 2 or AAV2, to deliver a healthy copy of the DDC gene to nerve cells. The treatment is expected to increase the production of a working AADC enzyme in cells, which in turn will increase levels of serotonin and dopamine to help ease disease symptoms.
Who can take Kebilidi?
Kebilidi was granted accelerated FDA approval in November 2024 for the treatment of adult and pediatric patients with AADC deficiency across the full spectrum of disease severity.
The FDA gives accelerated approval to treatments that show early evidence of being effective, but where long-term efficacy has not been definitively confirmed.
Its accelerated approval was based on clinical trial data showing that Kebilidi could ease AADC deficiency symptoms in the first year following treatment. Continual FDA approval is contingent on longer term trial data as well as subsequent clinical studies.
This decision made Kebilidi the first treatment for AADC deficiency to be granted FDA approval and the first gene therapy approved in the U.S. that is directly administered to the brain.
In the U.K. and EU, the gene therapy is marketed as Upstaza and has been approved since 2022 to treat AADC deficiency in patients ages 18 months and older.
Who should not take Kebilidi?
Kebilidi should not be administered to patients who have not yet achieved full maturation of the skull, as assessed by neuroimaging. The surgical procedure used with Kebilidi cannot be safely performed in those who have not reached full skull maturity, a term referring to the development and hardening of skull bones.
How is Kebilidi administered?
Kebilidi is available as a clear to slightly opaque, colorless to faint white liquid in single-use vials that contain 280 billion vector genomes (vg) — which are individual particles of the therapeutic virus — of the gene therapy in 0.5 milliliters (mL) of liquid.
Kebilidi is administered directly into the putamen, the area of the brain with the highest AADC activity and where dopamine signaling is key for motor, cognitive, and emotional functions. This is done via a minimally invasive neurosurgical procedure called stereotactic brain surgery in a specialized medical center.
Prior to surgery, patients need to undergo brain imaging so that doctors can plan for exactly where to administer the gene therapy.
The gene therapy is given via four infusions, two on the right side of the putamen and two on the left, using an FDA approved device called the SmartFlow Neuro Cannula. These infusions are done in a single neurosurgical procedure.
Each infusion delivers 45 billion vg of gene therapy, for a total dose of 180 billion vg. Each infusion takes about half an hour, and each needs to be performed using an infusion pump capable of delivering the therapy at a rate of 0.003 mL per minute.
Kebilidi in clinical trials
The FDA’s decision to give accelerated approval to Kebilidi was based on data from a Phase 2 trial (NCT04903288) of the therapy in 13 children with AADC deficiency. Patients ranged in age from just older than 1 year to almost 11.
Prior to receiving the gene therapy, 12 of these 13 children had a severe type of AADC deficiency, meaning that they had not achieved any of the motor milestones usually seen in the first year of life, and had no clinical response to standard of care therapies. One child was able to sit up with assistance, but had not achieved head control.
Kebilidi’s efficacy in this study was assessed using a standard measure of motor development called the Peabody Developmental Motor Scale, Second Edition (PDMS-2). Of the 13 participants, 12 remained in the study for at least 48 weeks (about one year). Evaluation at this time showed eight of the 12 children reached new motor milestones: three achieved full head control, two were able to sit with or without assistance, and two were able to walk backwards.
The eighth patient, able to sit with assistance prior to gene therapy, was able to sit up unassisted following treatment. For context, natural history data from 43 patients with AADC deficiency showed none had achieved new motor milestones in the absence of treatment.
The four patients who did not achieve any new motor milestones were generally older at gene therapy treatment, ranging in age from 2.8 to 10.8 years.
The Phase 2 trial is ongoing and expected to run through 2028.
Other trials and compassionate use studies
Kebilidi also was tested in a Phase 1/2 clinical trial called AADC-010 (NCT01395641) and a Phase 2b trial dubbed AADC-011 (NCT02926066), both conducted in Taiwan, as well as in two compassionate use studies, one each in Taiwan and Europe.
Data from these studies have broadly shown that children with AADC deficiency treated with the gene therapy experienced improvements in motor function, including the capacity to stand and sit with support — motor milestones rarely achieved by these patients — as well as head control and being able to reach or grab objects.
Pooled analyses from the Phase 1/2 and Phase 2 clinical trials and the Taiwan compassionate use study showed that, for 26 children who completed at least one year of follow-up, the average PDMS-2 score significantly increased, by more than 70 points, at one year after Kebilidi treatment.
Significant reductions in the severity of oculogyric crises — a common eye movement disorder in AADC deficiency patients — also were observed after one year, along with significant cognitive and language improvements.
These gains were sustained for up to five years, with children meeting new motor milestones years after treatment. Such milestones included walking without help or running freely. Further motor development also was observed during five to 10 years of follow-up in the four children who were followed for up to a decade. Patients who were younger at treatment administration generally showed the most dramatic long-term improvements.
Kebilidi is a recently developed therapy and the patients treated in the earliest clinical trials now are teenagers, so its longer term outcomes aren’t definitively known yet. Nonetheless, models have suggested that the treatment is likely to substantially improve life expectancy and quality compared with what would be expected for untreated patients.
Common side effects of Kebilidi
The most common side effects of Kebilidi include:
- dyskinesia (uncontrolled movements)
- fever
- low blood pressure (hypotension)
- anemia (low red blood cell counts)
- excessive salivation
- abnormally low levels of certain minerals including calcium, phosphate, and/or magnesium
- insomnia
- complications from the surgical procedure used for administration.
Procedural complications
Complications from the surgery used to administer Kebilidi have been reported. These can include leaking of the fluid that surrounds the brain, bleeding or inflammation in the brain, tissue death due to poor oxygen supply (infarction), and infections. There also have been cases of respiratory and cardiac arrest (meaning the heart stops beating or patients stop breathing).
In light of these potential risks, patients need to be carefully monitored, including close monitoring of heart and lung function, after the surgical procedure used to deliver Kebilidi.
Dyskinesia
One of the most common side effects of Kebilidi is dyskinesia, referring to uncontrolled movements that may look like fidgeting, writhing, wriggling, head bobbing, or body swaying. In clinical trials, patients who developed dyskinesia did so within three months of gene therapy administration, and there were some cases where dyskinesia was severe enough to require hospitalization. Dyskinesia in these cases was transient, reported to resolve three to seven months later.
Patients should be monitored for signs of dyskinesia following treatment with Kebilidi. If this side effect develops, it may be managed with dopamine antagonists (medications that block dopamine).
Use in pregnancy and breastfeeding
No data exist on the use of Kebilidi in people who are pregnant or breastfeeding. Animal studies in these situations have not been conducted. Kebilidi should not be administered to patients who are pregnant.
AADC News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare providers with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Statement backing EU patient representation gains more support
- We’re grateful our daughter quickly recovered from COVID-19
- New PromoterAI algorithm may aid rare disease diagnoses
- Finding the right words to describe what it means to be a father
- PNRI podcast explores research into rare diseases like AADC deficiency
Related articles