Celebrating Our Daughter’s ‘Reborn Day,’ Thanks to Gene Therapy
A columnist honors the day his daughter had gene therapy for AADC deficiency
Written by |

My wife and I enrolled our daughter, Rylae-Ann, who has aromatic l-amino acid decarboxylase (AADC) deficiency, in a one-year clinical trial that required her to stay in Taiwan. We had accepted new jobs in Singapore, so Rylae-Ann’s grandma and nanny stayed with her the entire time while we took turns flying back to be with her.
On Nov. 13, 2019, my wife, Judy, scheduled her visit to coincide with Rylae-Ann’s experimental gene therapy treatment. I had to nervously wait and watch by video call in Singapore. We weren’t sure what the results would be. All we knew was that our daughter was routinely submitted to emergency care, wasn’t developing, and was failing to thrive. If we didn’t act, we would lose her.
This day would become known to us as the day Rylae-Ann was reborn.
The day after
Rylae-Ann awoke the day after surgery. She was groggy and irritable, but there was sporadic movement. The doctor told us to expect this uncontrolled movement, known as dyskinesia, as a side effect. However, it was moving to us and looked like more than just a side effect. We did our best to keep our excitement and expectations in check. Instead, we focused on caring for her.
We began early intervention once she was discharged from the hospital a few days later. She still had stitches on the top of her head, so we didn’t push her too much. No one was telling us to start early intervention, but no one was telling us it wasn’t a good idea, either. As educators, we place a considerable emphasis on early intervention, so we began immediately.
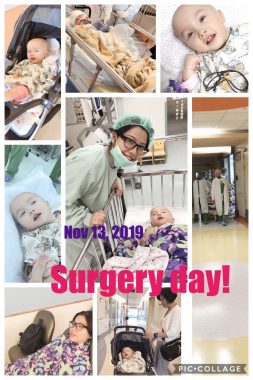
Rylae-Ann has gene therapy treatment on Nov. 13, 2019, which marked the start of a new tradition we call “reborn day.” (Courtesy of Richard E. Poulin III)
Core exercises
The early intervention consisted of physical therapy exercises that focused on her core. We continued her other therapies as we had done before gene therapy, but physical therapy was minimal due to her hypotonia, or decreased muscle tone, and immobility.
Following gene therapy, we wanted to maximize the potential results. As the brain-infused therapy began to do its thing, we wanted to get her body ready. It was not easy starting this new process, but we worked as a team to push her through her therapy sessions.

After gene therapy, Rylae-Ann begins physical therapy to maximize the results of the treatment. (Courtesy of Richard E. Poulin III)
Creating unity
During the clinical trial, we couldn’t talk to other participants, so we were unsure of what they were doing. Also, we didn’t speak publicly about our daughter’s progress or the results. We were in a bubble and relied on limited published research and resources from other rare disease organizations to guide us.
At the end of the clinical trial, we began to open up and share. We reached out to other families to learn about what they were doing. It was wonderful to see that they were employing similar strategies. It reassured us that we were on the right track. When we learned new strategies, we immediately incorporated them.
This gave us the idea to create a post-gene therapy Facebook group to foster the conversations we were having individually. Although it is a private group, it is open to anyone that would like to learn. We reach out to families that have undergone gene therapy, regardless of disease or method. The work our families do now will provide more evidence and guidance to future families on how they should proceed before and after.

Rylae-Ann and her parents celebrate her “reborn day” by singing “Happy ‘GT’ Birthday.” (Courtesy of Richard E. Poulin III)
A community tradition
From our Facebook group and meet-ups with other families, we discovered that we all shared the idea of celebrating our child’s “reborn day.” I was not surprised that all families celebrated this momentous occasion, but it was unique that we all called it a day of rebirth. Even Paul Wuh-Liang Hwu, from National Taiwan University Hospital, who led the clinical trial of eladocagene exuparvovec and was mentioned in a recent interview with New Scientist, acknowledges that, “We say they have been reborn.” The results have been dramatic for our children and have led them to a second chance at life.
This reborn day was extra special for us. Rylae-Ann has been three years post-gene therapy, which means she has now lived most of her life after receiving treatment.
As I said in a column celebrating my one-year anniversary with Bionews, the parent company of this website, it is important that I still create support for families who have not yet received treatment. We will not stop until everyone celebrates reborn day in our AADC deficiency community.

(Courtesy of Richard E. Poulin III)
Note: AADC News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of AADC News or its parent company, Bionews, and are intended to spark discussion about issues pertaining to aromatic l-amino acid decarboxylase deficiency.







Leave a comment
Fill in the required fields to post. Your email address will not be published.