We Must Reduce the Wait for AADC Deficiency Diagnosis and Treatment
Written by |

Our daughter, Rylae-Ann, was born in April 2018. By December of that year, she had been diagnosed with aromatic l-amino acid decarboxylase (AADC) deficiency, and we had received a subsequent verbal agreement to join a gene therapy clinical trial the next year.
The eight-month wait while trying to determine the disease my daughter was experiencing and the 11-month wait for therapy felt never-ending. Each day seemed like a wasted moment, a lost opportunity to make memories, and another missed milestone. However, our family’s story is swift compared with most in our community.

Richard, Judy, and Rylae-Ann pause for a holiday snapshot during a trip to Taiwan in 2018. After Rylae-Ann was diagnosed with AADC deficiency, the family was offered the option to participate in a gene therapy clinical trial. (Photo by Richard E. Poulin III)
Why the wait?
Although our duration was much shorter than that of others, the pain was just as intense. Discussion of waiting in our community is filled with frustrated tones and exhalations of irritation. These negative feelings seem to be growing as more data highlight the success of gene therapy. When we know an option is available, why must our children be forced to wait? Why must they continue to suffer when science shows that a completely different life is within our reach?

Waiting for gene therapy was difficult, but Rylae-Ann’s parents did their best to make her happy and healthy in the meantime. (Photo by Richard E. Poulin III)
Right to Try
Without treatment, many children cannot cope with the life-threatening symptoms of AADC deficiency. Life expectancy is incredibly short, and this should make our children fall under the Right to Try Act, signed into law in the U.S. in 2018.
Right to Try offers terminally ill patients who do not have any government-approved options a pathway to potential treatments under study. With regular obituary postings throughout the year of children succumbing to this disease, families should be given a chance to try an experimental treatment.
The week after we found out Rylae-Ann would be able to have gene therapy, she was admitted for pneumonia. Her face was pale, and she lay crumpled in our arms, more limp than usual. Judging by the doctors’ faces, we knew that this hospital admission was the most serious to date. After receiving word that our daughter could be treated, there she was fighting for her life.
She was finally discharged after a week, but the wait continued. That wait included fighting to stay alive and remaining eligible for treatment in the clinical trial. If her health wasn’t well, her gene therapy appointment could be postponed or, worse yet, canceled.
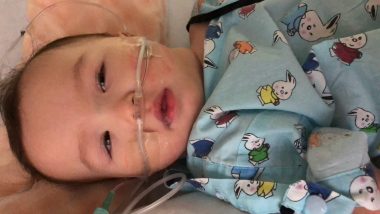
Rylae-Ann is admitted to the hospital for pneumonia after her parents learn she could receive gene therapy. (Photo by Richard E. Poulin III)
Emergency use authorization
Until the COVID-19 pandemic happened, I had never heard of emergency use authorization, a tool at the U.S. Food and Drug Administration’s disposal. The purpose of this regulatory step caught my attention following the approval of mRNA vaccines. By the start of 2020, the world was on notice about the new COVID-19 virus. By the end of that year, some countries had already begun approving vaccines for emergency use, giving rise to its description as a vaccine in a year.
By comparison, AADC deficiency was first identified in 1990. More than 30 years later, there is no approved treatment, and clinical trials are still being conducted to meet the requirements of various international regulatory bodies, all of whom have wonderful intentions of protecting those they serve.
Emergency use authorization helps to expedite the availability of treatments during a public health emergency. AADC deficiency does not fall into the same category as a pandemic or public health emergency, but it shows the importance of having a pathway for particular circumstances.
Additionally, gene therapy data show that the potential benefits outweigh the risks associated with surgery for AADC deficiency. While clinical trials continue and the approval process takes its ordinary course, families should be given an expedited pathway to treatment. The COVID-19 pandemic has slowed the process and added more wait time for our community.

The results of gene therapy clinical trials give hope to many in the AADC deficiency community. (Photo by Richard E. Poulin III)
Hope
Our experience, which I often refer to as “us skipping the queue,” brought treatment much sooner than most in our community. However, the wait was still painful. This pain is even greater for others. Since 1990, 135 cases of AADC deficiency have been described worldwide, but the true number could be as many as 1,800 cases. All are waiting while they desperately search for some saving grace.
I know we do what we must without hesitation, but the feeling of watching our children suffer is daily torture until they are given help. My hope is that the wait will be significantly reduced. This is a real possibility with new testing options to speed up the diagnosis process and with access to future treatment options on the horizon.
Note: AADC News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of AADC News or its parent company, Bionews, and are intended to spark discussion about issues pertaining to aromatic l-amino acid decarboxylase deficiency.






Leave a comment
Fill in the required fields to post. Your email address will not be published.