I’m hopeful about the future of gene therapy for AADC deficiency
Upstaza's forthcoming availability in England is a good sign, this writer says
Written by |

My wife, Judy, and I had stayed up late for meetings and written papers advocating for the approval of Upstaza (eladocagene exuparvovec), so we celebrated when the news was finally made public. In November, the gene therapy formerly known as PTC-AADC was approved in the U.K. to treat patients ages 18 months and older with severe aromatic l-amino acid decarboxylase (AADC) deficiency. This followed on the heels of the European Commission’s approval a few months prior.
Now, in addition to those approvals, England’s National Institute for Health and Care Excellence (NICE) has recommended in its final draft guidance, published on March 23, that Upstaza be available to AADC patients at little to no cost.
According to AADC News, “The favorable decision … makes Upstaza the first disease-modifying treatment for AADC deficiency added to the list of medications available through U.K.’s National Health Service (NHS), a public health program funded by taxpayers.”

Judy and Richard traveled to Europe to share a parent perspective on Upstaza and raise awareness of AADC deficiency in October. (Courtesy of Richard E. Poulin III)
What this means for our community
Last May, I wrote that Upstaza’s approval could open the door for more competition and innovation in the area of treatments. I believe NICE’s recommendation will have the same effect.
Researching and developing new therapies is a costly undertaking, and it can be risky, as there’s no guarantee that a therapy will be approved. Pharmaceutical companies may hesitate to invest in ultrarare diseases like AADC deficiency since the patient population is so small. But the NHS adoption of this medical breakthrough could encourage further investment in this area of medical science.
I’m hopeful that other gene therapies will become more available and common, especially since my daughter, Rylae-Ann, had such a positive experience with Upstaza. Treatment options for AADC deficiency are minimal, so Judy and I enrolled her in a clinical trial in 2019, in which she received the one-time therapy. The benefits have been life-changing.
NICE’s recommendation reflects this, with the committee agreeing that Upstaza has been proven to offer “transformative” and “substantial” benefits for AADC patients.
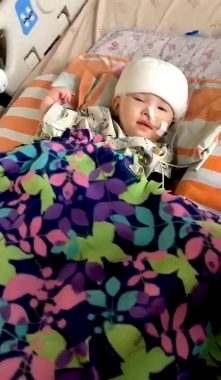
Rylae-Ann undergoes gene therapy during a clinical trial in Taiwan in November 2019. (Courtesy of Richard E. Poulin III)
Lowering costs
Although gene therapy can be life-changing, it often comes with a significant price tag, raising the question of how patients will be able to afford it. For example, Hemgenix (etranacogene dezaparvovec), the first gene therapy for adults with hemophilia B, was approved by the U.S. Food and Drug Administration last November. But as Bloomberg Law reported, the one-time therapy comes with a price tag of $3.5 million per dose, making it the most expensive drug in the world.
NICE’s recommendation that Upstaza be available at little to no cost addresses this issue for a handful of patients in England, but increased competition and innovation in the area of treatment could provide another solution for patients in other countries.
When companies are forced to compete for customers, it can result in lower prices, higher quality, improved service, more variety, and greater innovation. The medical science and pharmaceutical industries are no exception.
Over time, additional gene therapies may become available, leading to a decrease in price. Unfortunately, time is not on our side, as many children with AADC deficiency die in early childhood. Perhaps different therapies or medications may become available sooner to supplement or provide an alternative to gene therapy.
It’s important to remember that innovators face the greatest risk and cost. PTC Therapeutics, the developer of Upstaza, has proven that there’s a market for AADC deficiency treatments. Hopefully, other pharmaceutical companies will now feel more confident following suit.
In the meantime, Judy and I will continue pushing for Upstaza’s approval in other countries, with our next goal being the U.S. And in addition to speaking and writing initiatives, our daughter is enrolled in a long-term study on the effectiveness of Upstaza so that we may continue to contribute data and demonstrate the long-lasting effects of the therapy.
Note: AADC News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of AADC News or its parent company, Bionews, and are intended to spark discussion about issues pertaining to aromatic l-amino acid decarboxylase deficiency.






Leave a comment
Fill in the required fields to post. Your email address will not be published.